近日,半岛平台材料科学与工程学院陈江照和易健宏团队在国际化学顶级期刊Angewandte Chemie International Edition上以“Polydentate ligand reinforced chelating to stabilize buried interface toward high-performance perovskite solar cells”为题发表文章,该研究工作得到国家自然科学基金面上、兵团重点领域科技攻关计划项目等项目的资助。
金属卤钙钛矿太阳能电池因其优异的性能在光伏领域引起了广泛的关注。目前已经实现了高达26.1%的认证效率,该效率能够与单晶硅电池的效率媲美。然而,差的长期工作稳定性对钙钛矿光伏技术的商业化提出了严峻的挑战。器件中每一个功能层及其界面与电池的长期稳定性密切相关。其中,正式p-i-n电池的埋底界面对制备高效稳定钙钛矿太阳能电池至关重要。
鉴于此,陈江照教授和易健宏教授团队开发了一种多齿配体增强的螯合策略,通过管理界面缺陷和应力来提高埋底界面的稳定性。采用双(2,2,2-三氟乙基)(甲氧羰基甲基)膦酸酯(BTP)修饰埋底界面。BTP中的C=O、P=O和两个-CF3官能团协同钝化SnO2表面和钙钛矿薄膜底表面的缺陷。而且,BTP修饰有助于减轻界面残余拉应力,促进钙钛矿结晶,降低界面能垒。该多齿配体调控策略适用于不同的钙钛矿组分,具有很好的普适性。由于显著的减少了非辐射复合和显著提高的界面接触,BTP修饰的器件实现了24.63%的功率转换效率(PCE),这是空气环境制备的器件报道的最高效率之一。未封装的BTP修饰的器件在10-20%RH环境中老化3000小时以上保持初始效率的98.6%。未封装的BTP修饰的器件加热老化1728小时后保持初始效率的84.2%。本工作为通过设计多齿螯合配体分子增强埋底界面稳定性提供见解与指导。
陈江照教授长期从事新能源材料与器件研究,共发表SCI论文104篇,总引用8300余次,H指数为34。其中,以第一或通讯作者发表SCI论文82篇,包括1篇Nat. Energy、7篇Adv. Mater.、1篇Energy Environ. Sci.、2篇Angew. Chem. Int. Ed.、4篇Adv. Energy Mater.、4篇Adv. Funct. Mater.、3篇ACS Energy Lett.、1篇Nano-Micro Lett.、2篇Nano Lett.、2篇Nano Energy等,ESI高被引论文19篇,ESI热点论文4篇,单篇论文最高引用490余次,单篇引用超过100次的论文有16篇,1篇论文入选ACS Energy Letters亮点文章。申请发明专利12项,其中获授权7项。作为主编出版中文专著4部。主持国家自然科学基金面上、兵团重点领域科技攻关计划项目、重庆市自然科学基金面上、重庆市留学人员回国创业创新支持计划重点项目等科研项目8项。获得2023年全球前2%顶尖科学家、新疆天池英才特聘教授、半岛平台拔尖人才(三层次)、重庆大学百人、第二届沙坪坝区十佳科技青年、2023川渝科技学术大会优秀论文特等奖、重庆市科协岗位创新争先行动三等奖、第三届川渝科技学术大会优秀论文二等奖、2022年Wiley威立中国开放科学高贡献作者奖、2022年Wiley威立中国开放科学年度作者奖等奖励与荣誉10余项。在国内外重要学术会议作邀请报告近20次。担任国际/国内学术会议大会主席(1次)、大会秘书长(1次)和分会场主席(4次)。担任Nature、Nat. Rev. Phys.、Joule等40余本国际知名学术期刊的审稿人。担任Nano-Micro Lett.、Carbon Energy、SmartMat、Nano Mater. Sci.、Sci. China-Mater.、eScience和Carbon Neutrality期刊的青年编委及先进储能材料与技术兵团重点实验室学术委员会委员。
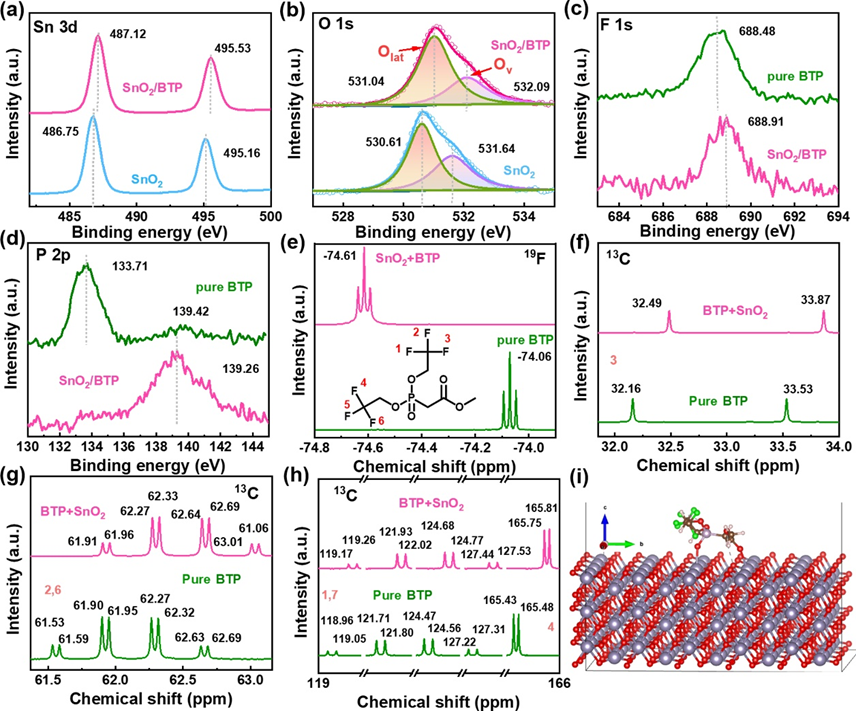
Figure 1.(a) Sn 3d, (b) O 1s, (c) F 1s and (d) P 2p XPS spectra of the SnO2and SnO2/BTP films. (e)19F NMR, and (f-h)13C NMR spectra of the SnO2solutions without and with BTP. (i) TheBinding energies (Eb) between the OVdefects in SnO2in contact with theBTPmolecule. Optimized structure of SnO2surface containing OVdefects.
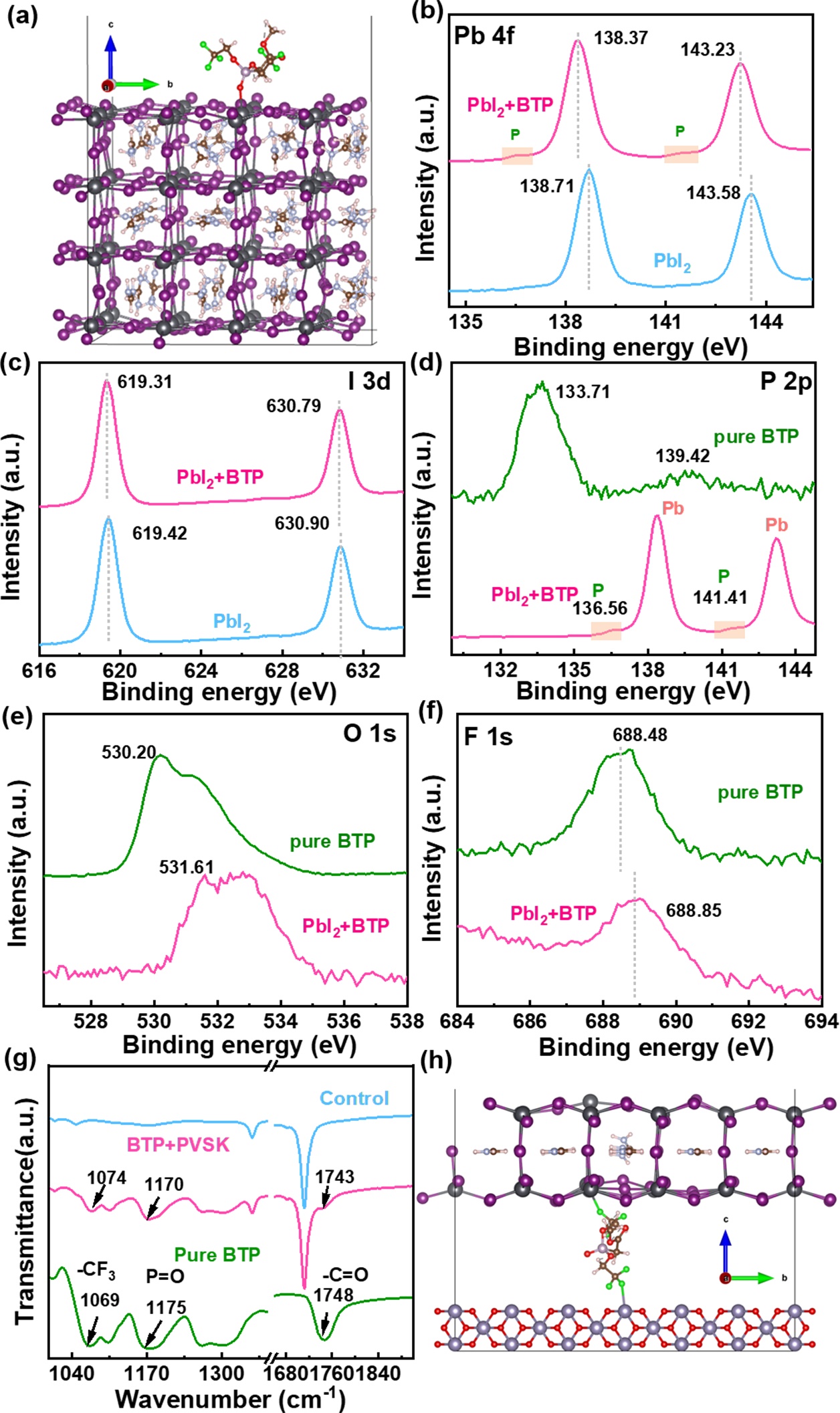
Figure 2.(a) Optimized structures of FAPbI3surface containing iodine vacancy defects with BTP. (b) Pb 4f, (c) I 3d, (d) P 2p, (e) O 1s and (f) F 1s XPS spectra of the pure BTP, PbI2and PbI2+BTP films. (g) FTIR spectra of the perovskite, BTP+perovskite, and pure BTP films in the range of 1000-1900 cm-1. (h) The relaxed structure of BTP molecules bridging SnO2substrate and perovskite through chemical bonds.

Figure 3.AFM imagesof the SnO2films (a) without and (b) with BTP. (c) XRDpatterns and (d) XRD intensity and FWHM for the control and BTP-modified perovskite films. GIXRD spectra with different ω values (0.5~1.5) of the (e) control, and (f) BTP-modified perovskite films.
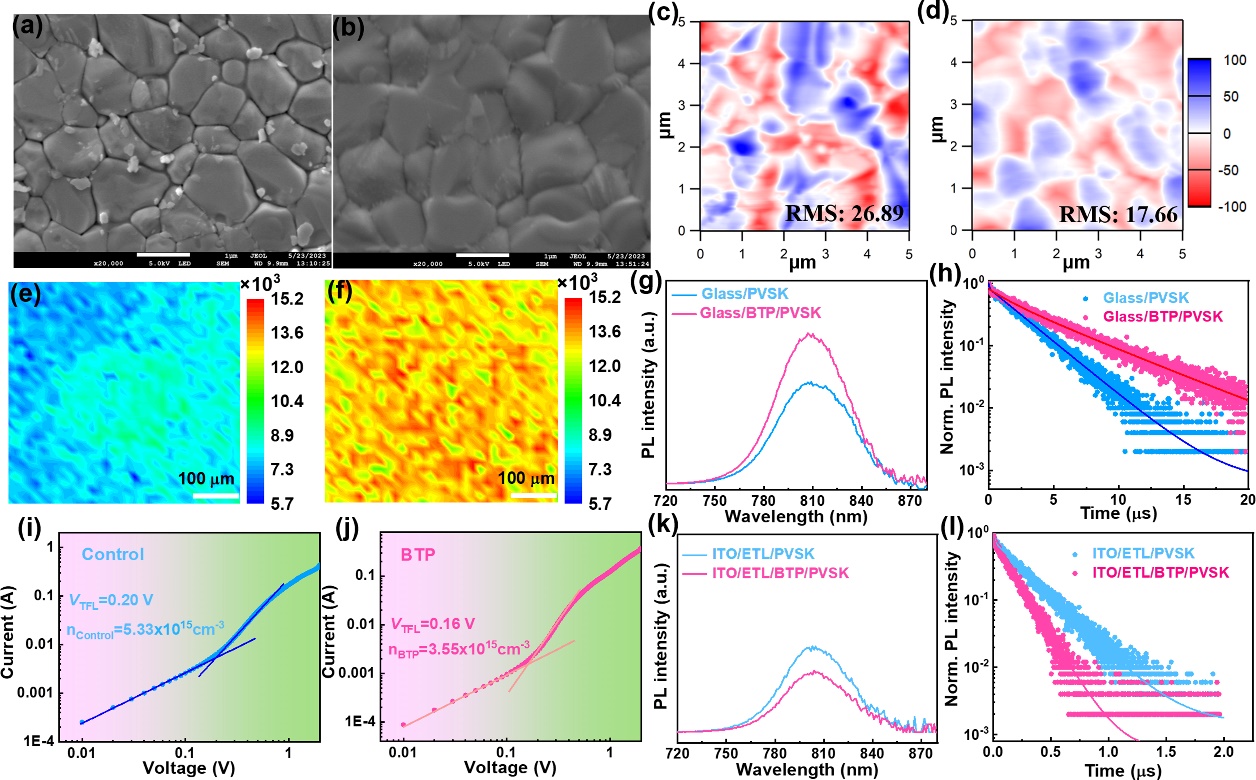
Figure 4.Top-view SEM images of the (a) control and (b) BTP-modified perovskite films. The scale bar is 1 μm.AFM images of the perovskite films (c) without and (d) with BTP modification. PL mapping images of the (e) glass/perovskite and (f) glass/BTP/perovskite films. (g) SSPL and (h) TRPL spectra of the glass/without and with BTP/perovskite films measured from the glass side. PVSK stands for the perovskite layer. SCLC for the electron-only devices with the structure of the (i) ITO/SnO2/perovskite/PCBM/BCP/Ag and (j) ITO/SnO2/BTP/perovskite/PCBM/BCP/Ag. (k) SSPL and (l) TRPL spectra of the perovskite films deposited on the SnO2substrates without and with BTP modification.
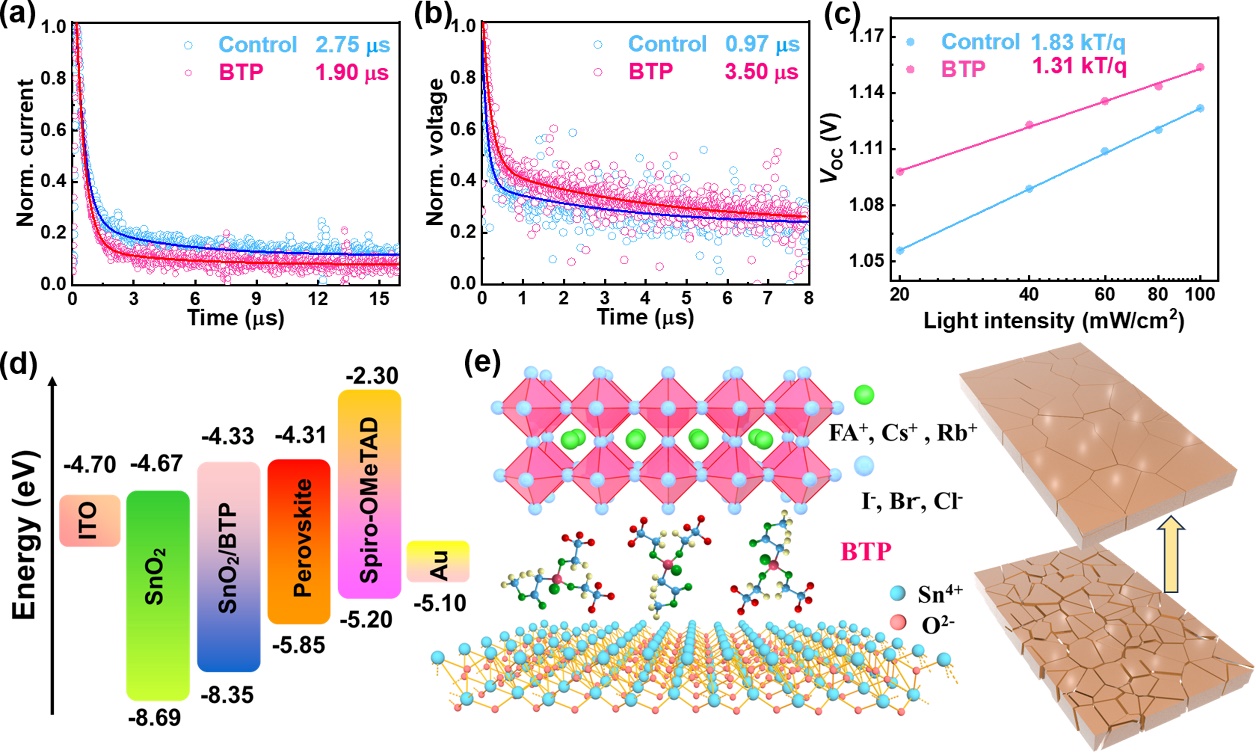
Figure 5.(a) TPV and (b) TPC decay curves of the PSCs without and with BTP. (c) The light-intensity dependence ofVOCcurves for the control and BTP-modified devices. (d) Energy level diagram of calculated SnO2and SnO2/BTP in comparison with the energy levels of ITO, perovskite films, Spiro-OMeTAD (HTL) and Au. (e) Schematic of the buried interface modified by polydentate ligand BTP.
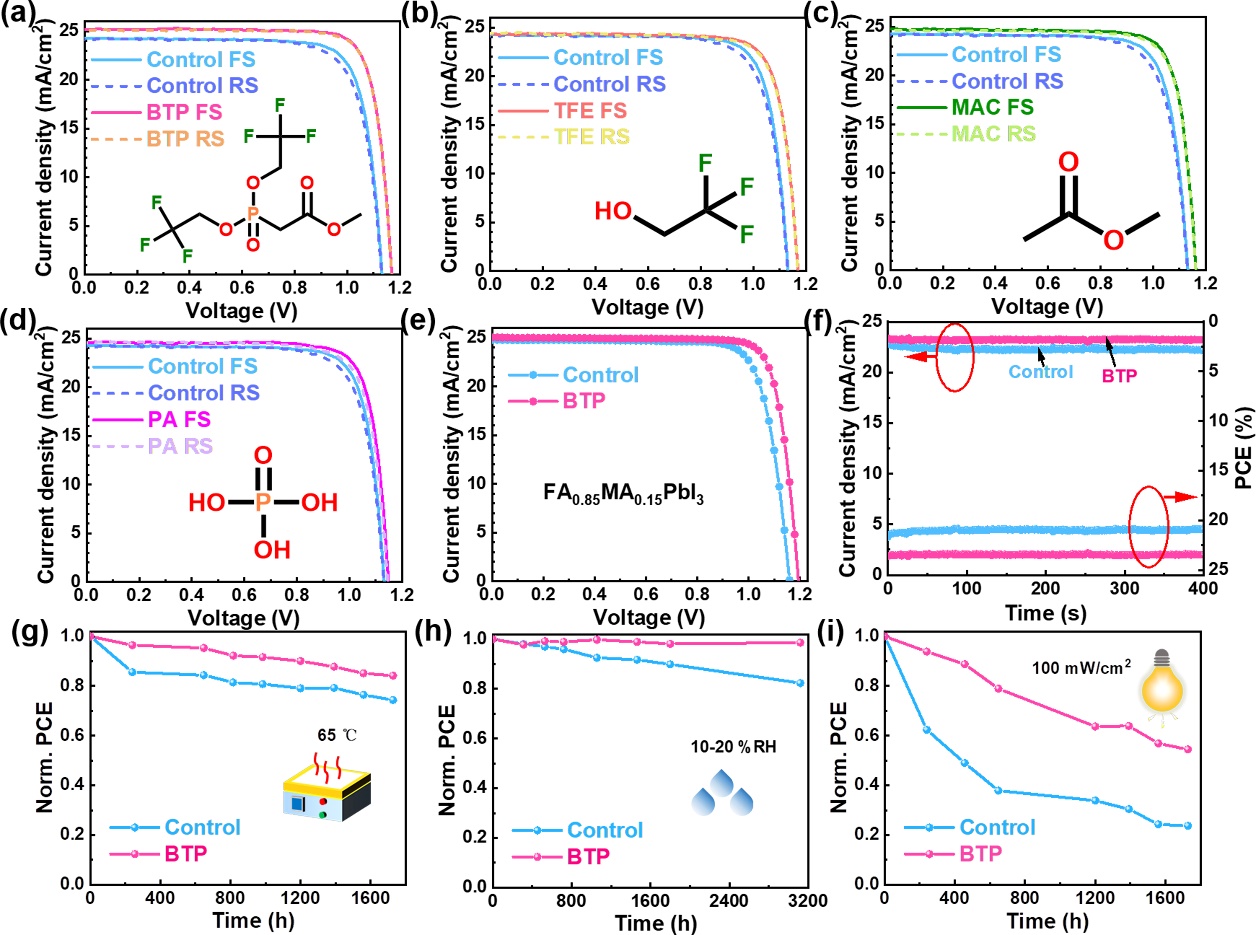
Figure 6.(a)J-Vcurves of the champion control and BTP modified devices. (b)J-Vcurves of the devices without and with TFE. (c)J-Vcurves of the devices without and with MAC. (d)J-Vcurves of the devices without and with PA. (e)J-Vcurves of best-performing devices without and with BTP using FA0.85MA0.15PbI3composition.(f) The stabilized photocurrent density of best-performing devices without and with BTP using Rb0.02(FA0.95Cs0.05)0.98PbI2.91Br0.03Cl0.06composition. (g) The stability of the PSCs without and with BTP heated at 65 ℃ in the dark in an N2-filled glovebox. (h) The stability for the control and BTP-modified devices under a relative humidity (RH) of 10-20% in the dark. (i) The PCE evolution for the control and BTP-modified devices under one sun illumination of 100 mW/cm2provided by white light LED at room temperature in the N2-filled glovebox.
文章链接:
Baibai Liu#, Qian Zhou#, Yong Li#, Yu Chen, Dongmei He*, Danqing Ma, Xiao Han, Ru Li*, Ke Yang, Yingguo Yang, Shirong Lu, Xiaodong Ren*, Zhengfu Zhang, Liming Ding, Jing Feng, Jianhong Yi*, Jiangzhao Chen*. Polydentate ligand reinforced chelating to stabilize buried interface toward high-performance perovskite solar cells.Angewandte Chemie International Edition2024,e202317185.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202317185
(供稿:材料学院)
 滇公网安备53011402000430号 All Rights Reserved © Kunming University of Science and Technology
滇公网安备53011402000430号 All Rights Reserved © Kunming University of Science and Technology